Technology
Infiniplex Technology
Sampling
Extracting
Analyzing
Testing
Sampling
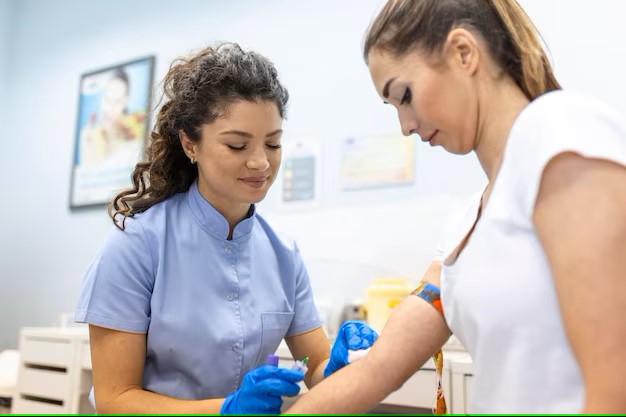
Infiniplex offers a cutting-edge approach to healthcare through Liquid Biopsy. The initial step in our liquid biopsy process is the collection of a simple blood sample.
This sample enables us to collect circulating biomarkers from a patient’s bloodstream, such as cell-free DNA (cfDNA) and circulating tumor DNA (ctDNA). This method allows for the assessment of genetic mutations without requiring invasive tissue biopsies. Liquid biopsies are gaining prominence in oncology as a valuable tool for early cancer detection, monitoring treatment responses, and detecting minimal residual disease, offering a less invasive and more accessible approach to understanding a patient’s disease status.

Extracting
Cell-free DNA (cfDNA) is extracted from the collected blood sample. This step allows us to isolate the genetic materials within the blood for later analysis, will provide valuable insights into the patient’s health and help us detect diseases in their earliest stages.
Analyzing
After the precise extraction of cell-free DNA (cfDNA) from the blood sample, the lab employs a powerful and prevalent analytical method known as quantitative Polymerase Chain Reaction (qPCR). This technique amplifies and quantifies specific DNA sequences within the extracted cfDNA. Using our proprietary multiplexing method allows us to amplify dozens of DNA targets within a single reaction simultaneously. What sets our approach apart is our unique primers, carefully designed to target dozens of specific cancer mutations associated with various cancer diseases. Through these innovative primers, we can simultaneously and selectively detect and measure the presence of a wide range of genetic mutations. This detailed analysis allows for early disease detection, treatment monitoring, and the development of highly personalized healthcare strategies. The decoding of the parameters produced during the qPCR process is automatically transferred to the processing and analysis of the results.


Interpreting
Our proprietary digital signal processing method allows for both quantifying the detected mutated sequences and accessing their clinical significance in accordance with clinical requirements.
Frequent follow-up testing allows for the assessment of disease progression.
Interpreting disease progression between consecutive tests significantly improves accuracy and reliability – by thoroughly examining the data to identify and understand any observable patterns, trends, or anomalies that have clinical significance for the patient’s health. Following this interpretation, the process moves towards formulating clear and concise conclusions. Determinations are made if results fall within normal ranges or if there are noteworthy concerns or anomalies that require immediate attention. After reaching these conclusions, the last step includes preparing a comprehensive report summarizing the entire process.
We have developed a decoding algorithm that detects the presence of a mutation/s from the dozens of tested mutations and a dynamic quantification algorithm that quantifies the detected mutations and provides dynamic tracking of the mutation/s load.

